Exploring Human Comedogenic Potential of Everyday Cosmetics through Cyanoacrylate Follicular Biopsy Analysis by Novobliss Research
5 January 2024
Cosmetic products are topical preparations widely used for cleansing, beautifying, promoting attractiveness or altering the appearance of body without affecting its structure or functions.
Researches have shown that the frequent use of several cosmetics is linked to formation of comedones and acne because these products typically contain ingredients such as artificial colours, oils, fragrances, and preservatives that can exacerbate acne-prone or oily skin. These comedogenic ingredients can lead to the accumulation of sebum, bacteria and dead skin cells within the pores, resulting in the formation of comedones (blackheads and whiteheads) and acne lesions, thereby influencing the overall health and appearance of the skin.
Products identified as non-comedogenic aim to minimize the risk of pore blockage, promoting clear and healthy skin. Non-comedogenic claims are frequently integrated into cosmetic marketing and labelling strategies. The execution of non-comedogenicity studies enables manufacturers to validate such claims, aligning their products with professional and regulatory standards. These studies play a crucial role in cultivating trust among consumers by providing scientific evidence that supports the safety of these products for the skin.
NovoBliss Research Private Limited, Gujarat, India, operating as a Contract Research Organization (CRO), specializes in claims substantiation for widely used cosmeceuticals, nutraceuticals, and FMCG products. For conducting non-comedogenicity studies, our focus lies in employing the well-known “Cyanoacrylate Follicular Biopsy Analysis” technique. These assessments involve the evaluation of skin conditions before and after one month of cosmetic product usage. Test products undergo comparison with a positive control (Isopropyl Myristate solution or equivalent with a high comedogenicity profile) and a negative control (Normal Saline) to determine their comedogenic potential.
In our non-comedogenicity studies, approximately 0.2 mL/g of the test products, positive control, and negative control are applied to the centre of semi-occlusive patches, which are then placed on 4 cm2 pre-marked test sites on the right and left regions of the upper back of the subjects. These patches are secured with hypoallergenic tape [Medi-Tape 320 (Paper Tape) or equivalent] to ensure close contact with the skin. The patches are applied three times a week on Monday, Wednesday, and Friday for four consecutive weeks and are removed after 48 hours, 48 hours, and 72 hours of application time, respectively. After patch removal, each application site is gently cleaned with distilled water. Thirty minutes (+5 minutes) post-patch removal, an experienced dermatologist assesses irritation using the Berger and Bowman Scale (7-point irritation scoring scale).
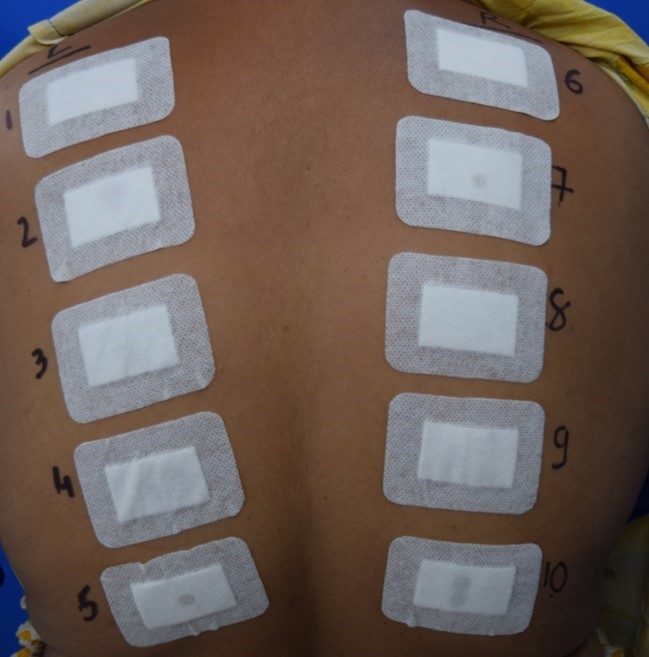
Cyanoacrylate Follicular Biopsy Analysis is conducted for all test sites before product application on day 1 (first visit) and 15 minutes after the last patch removal on day 29 (last visit) to compare microcomedones formation. Following staining of slides with Haematoxylin and Eosin, a highly experienced pathologist examines the biopsy slides under the microscope to count the microcomedones between the specified time intervals. Upon completion of counting, comedogenic scoring is performed based on the Follicular Biopsy Scoring Scale (0: non-comedogenic, 0.5: slightly comedogenic, 1: mild comedogenic, 2: moderate comedogenic, 3: strong comedogenic).
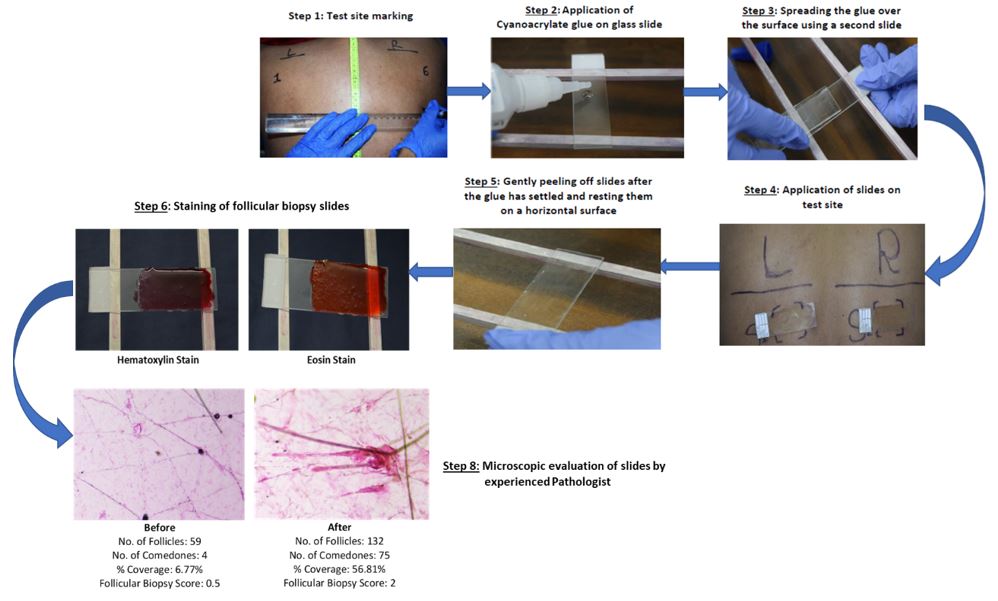
Through the application of this well-established technique, we ensure rigorous testing of all products for their comedogenic potential.
The increased prevalence of allergic contact dermatitis in children has prompted scrutiny of products marketed for neonates and children for the presence of sanitisers.
For more informations, visit the website : https://novobliss.in/.
*publirédactionnel
CONTACT
Maheshvari Patel: maheshvari@novobliss.in
Nayan Patel: Dr.Nayan@novobliss.in







 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.