Enhancing reliability in cosmetic clinical testing with Dermaclaim
14 March 2024
The use of before and after data in cosmetic product evaluations has long been a cornerstone of marketing and consumer decision-making, both for percentages and images. Images serve as visual subjective testimonials, demonstrating the potential transformative effects of skincare and cosmetic products, whereas the figures and percentages are useful to objectively show the improvement of the specific assessed biomarkers and validate the marketing claims regarding to before & after (B&A) status. However, controversies surrounding misleading claims and inconsistent representations have raised concerns about the reliability and credibility of such comparisons. To maintain accuracy and reliability, understanding and implementing best practices for conducting before and after clinical trials is imperative.
Best practices in before and after clinical trials
A crucial aspect of conducting before and after clinical trials is the establishment of standardized conditions and protocols. Without uniform conditions at the different timepoints for testing, data might not be valid, with the difference in the experimental conditions interfering in the analysis of results and conclusions:
- Consistency in lighting, background, and camera settings is vital to ensure uniformity in the images captured before and after product usage.
- Encouraging participants to adopt similar neutral poses and facial expressions helps accurately compare features in before and after photos. Avoiding exaggerated facial expressions is crucial, as these may misrepresent the true effects of the product.
- Using high-resolution and unaltered images, together with official colorcharts, ensures that the true representations of skin conditions or changes are depicted. Avoiding retouching or filters is crucial, as these can distort or enhance results beyond reality.
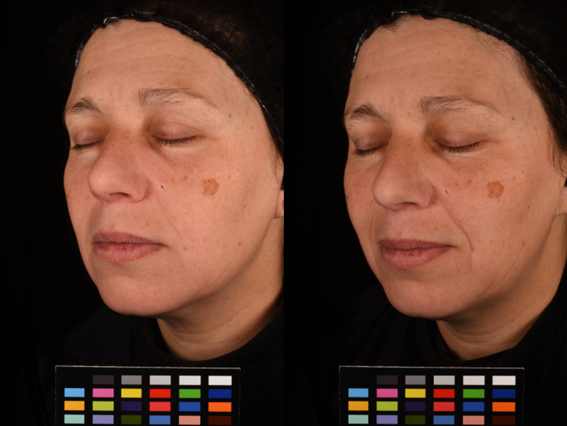
Figure 1. Ensuring uniform image conditions. Difference in the experimental conditions for image acquisition is one of the major problems in cosmetic clinical testing. In this example, we can observe small differences in lighting conditions and facial expression, comparing the before (left) and after (right), leading to misinterpretation and misleading conclusions.
- Regarding product application, establishing clear timeframes and protocols is essential. Results will be different whether the last application took place 2 hours or 12 hours before the technical measurements.
- Participants must adhere strictly to the application instructions. Assuring participants’ compliance with consumption controls, diaries, or other possible mechanisms such as a mobile application, is always useful to improve reliability of the results.

Figure 2. Mobile application to manage clinical tests in human volunteers. Screenshots obtained from the mobile application in development by Dermaclaim to manage volunteers’ participation and compliance in clinical tests.
- The utilization of reliable instrumental technologies also plays a pivotal role in substantiating the efficacy claims. Innovative and sophisticated instruments provide objective and quantitative measurements, enabling CRO to validate the effects of skincare formulations with precision and accuracy.

Figure 3. Using innovative technologies. Relying on innovative instrumental technologies such as 3D equipments (AEVA-HE with VisioTop 500 Bench is shown) improves the sensitivity and reliability of the findings, grounding results in empirical objective data and not only on clinical scores or subjective evaluations.
- Limiting the impact of external factors (temperature, relative humidity, acclimatization period, sun-derived natural variation of the skin…), both at the time of acquiring images and measurements in clinical facilities, and throughout the study period, is crucial.
- Obtaining explicit informed consent from participants before starting cosmetic clinical testing is crucial for ethical and legal reasons, both for participation and image usage.

Figure 4. Obtaining informed consent from participants.
- It is also important to consider obtaining the necessary permits and authorizations for the importation of cosmetic products and formulations. Before conducting efficacy clinical studies in human volunteers, products must have yielded positive results in safety clinical studies (Patch Test, HRIPT…) and the Cosmetic Product Safety Report (CPSR) must have been prepared.
- Last, and possibly the most important aspect of a clinical study in human volunteers, is the panel recruitment. The likelihood of obtaining positive results depends radically on the correct selection of the panel. Errors in establishing inclusion or exclusion criteria can mask the potential positive effect of a product, leading to incorrect interpretations and conclusions.
- Establishing a pre-treatment washout period where volunteers do not use any other products in the area of interest, with the goal of standardizing the volunteers’ baseline state as effectively as possible, is another recommended strategy before starting the treatment.
- Conducting thorough monitoring of each volunteer, identifying those who do not comply with protocols and are solely motivated by financial compensation, as well as avoiding individuals with medical problems, those considering a significant change in their living conditions (diet, exercise, stress, work, etc.), or individuals who have undergone relevant aesthetic interventions that may interfere with the effects of the treatment, are fundamental aspects to consider in the entire recruitment process. Likewise, training the volunteer panel with correct protocols for topical application can make the difference between obtaining or not obtaining significant results.
*publirédactionnel
CONTACT
Dermaclaim Lab S.L. ®
+34 96 187 45 82







 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.