Unlocking Precision in Dermatology Trials: The Impact of Optical Coherence Tomography for testing laboratories by VivoSight
20 May 2024
In the dynamic landscape of dermatology research and development, Clinical Research Organizations (CROs) play a pivotal role in driving innovation and advancing scientific understanding. Embracing cutting-edge technologies is not just an option but a strategic imperative for these organizations. Among these transformative tools, Optical Coherence Tomography (OCT) stands out as a game-changer, offering unique benefits that can revolutionize the way CROs conduct and manage claims validation trials.
 Subject friendly imaging
Subject friendly imaging
Recognising the need for CROs to flex analysis tools on a project by project basis, Michelson Diagnostics’ market leading VivoSight OCT system is now available to CROs through a flexible rental model.
Understanding Optical Coherence Tomography
Think of OCT imaging as the optical counterpart to ultrasound, employing an eye-safe laser. This completely non-invasive imaging modality enables high-resolution, 3D, cross-sectional visualization of skin structures to a depth of >1mm, allowing for detailed and quantitative analysis of treatment effects and tissue responses.
The quest for data
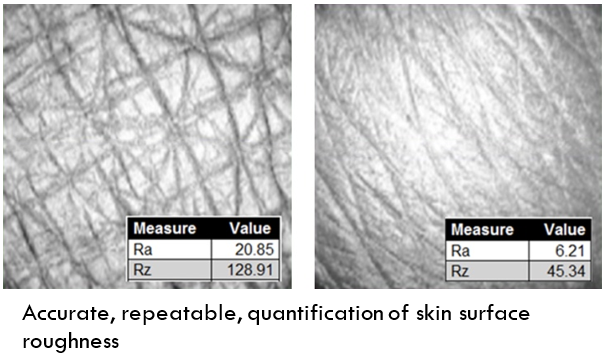
Quantitative testing of skin health and cosmetics treatments is becoming critical for claims validation, and OCT imaging can be a significant data enabler during the testing process. Michelson Diagnostics is at the forefront of OCT imaging system development and its VivoSight Dx system incorporates a comprehensive suite of quantitative analysis tools to transform skin images into valuable data. Automated quantitative measures include:
>Changes in collagen density, using Dermal Brightness proxy
>Epidermal thickness
>Skin surface roughness
>Vessel diameter and vascular density
Fast and friendly imaging
Real-time monitoring of skin dynamics during claims validation trials is crucial for understanding treatment mechanisms and refining protocols. Taking just 15 seconds to acquire a full 3D scan, VivoSight OCT imaging captures immediate skin responses following test-molecule interventions, facilitating informed adjustments and observations, thus accelerating research progress.
At the forefront of patient-centric imaging, OCT is always entirely non-invasive, uses no gel and offers a patient-friendly alternative to invasive procedures, minimizing discomfort and risk for study participants.
The Benefits of OCT imaging for CROs
1- Enhanced Research Precision: OCT provides comprehensive, quantitative, objective data on skin parameters, facilitating repeated precise evaluation of treatment efficacy and safety profiles, strengthening trial conclusions with regulatory authorities.
2- Market Leadership: Integrating OCT imaging sets CROs apart as leaders in quantitative, non-invasive, dermatology, attracting collaborations and clientele seeking advanced methodologies.
3- Flexible Financial Models: Recognising the growing pressure on trials budgets, VivoSight OCT is now available through a Rental Model, enabling project-based OCT-acquisition, and ensuring the most cost-effective scalability.
4- Streamlined Operations: VivoSight OCT incorporates project-efficiency tools such as batch-processing and simple data-export functions. Integration optimizes data acquisition and analysis processes, expediting trial timelines and enhancing project management efficiency.

In conclusion, Optical Coherence Tomography (OCT) presents a transformative opportunity for Clinical Research Organizations (CROs) in dermatology trials. With Michelson Diagnostics offering the VivoSight OCT system through flexible rental models, CROs can adapt analysis tools as needed. OCT’s quantitative analysis, real-time monitoring, and patient-friendly imaging enhance research precision, market leadership, financial flexibility, and operational efficiency. Embrace OCT to unlock a new era of precision and innovation in dermatology trials. Schedule your online product demonstration of the VivoSight Dx system and the VivoTools automated quantification suite.
Contact
Jon Holmes








 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.