Efficacy evaluation for mosquito repellent product by Claim
31 January 2025
CLAIM® is an Argentina-based Contract Research Organization (CRO) specializing in the design, development, and execution of clinical studies, leveraging advanced bioengineering methods. These studies are tailored to assess the safety and/or efficacy of a wide range of products, including cosmetics, pharmaceuticals, dermatological treatments, pet cosmetics, household products, and other topical applications.
With over 25 years of experience and commitment, we take pride in maintaining the highest standards of quality in the challenging Southern Cone region where we operate. By staying at the forefront of innovation and ensuring compliance with the latest international regulations, we help organizations worldwide achieve excellence in quality, safety, and compliance.
Still in the area of the world you are, mosquitoes repellents, as cosmetic or medical device, become an important product to help people in their daily life. Mainly concerns as dengue, yellow fever, chikungunya, Zika and discomfort drive the market to this kind of products for most laboratories worldwide.
The production of repellents is regulated by different types of health, environmental, consumer and trade authorities, which vary from region to region. Standards and requirements to ensure the safety, efficacy and quality of repellents are objectives that must be met when a product is placed on the market.
The protection time offered to the user from the moment of application is an essential parameter when defining the product and selecting it on the shelf. The study of efficacy gives an endorsement to the sustained claim.
THE TESTING FOR EFFICACY against Aedes aegypti
In line with our commitment to non-animal testing—one of CLAIM®’s core principles—and in collaboration with CIPEIN-UNIDEF-CONICET and CITEDEF, nationally renowned centers specializing in pest and insecticide research under the national state, we have developed a protocol to evaluate the efficacy of this type of product. CIPEIN, recognized as a GLP-certified ‘collaborating center’ by the World Health Organization, helps ensure that this initiative adheres to the highest standards of quality and control. This partnership allows us to align seamlessly with the World Health Organization’s recommendations while maintaining excellence at every step of the process.
For these tests we consider specific insect colonies raised in isolation and use cages designed to expose only a limited area of the panelists’ forearms, following established recommendations. We also account for variables that could influence insect behavior, including the test subjects’ dietary intake, clothing colors, and the presence of perfumes.
Time intervals at which the panelist exposes the skin to the insects and the responses to it are registered. A specific statistical analysis formula, Kaplan–Meier estimator, is applied.
Procedures and ethical aspects are aligned with WHO recommendations.
This procedure results in the determination of the CPT, Complete Protection Time associated with the tested product as an efficacy parameter.
These reports, beyond the statistical analyses, tables and the ‘Conclusions’ chapter, end up containing information such as the following:
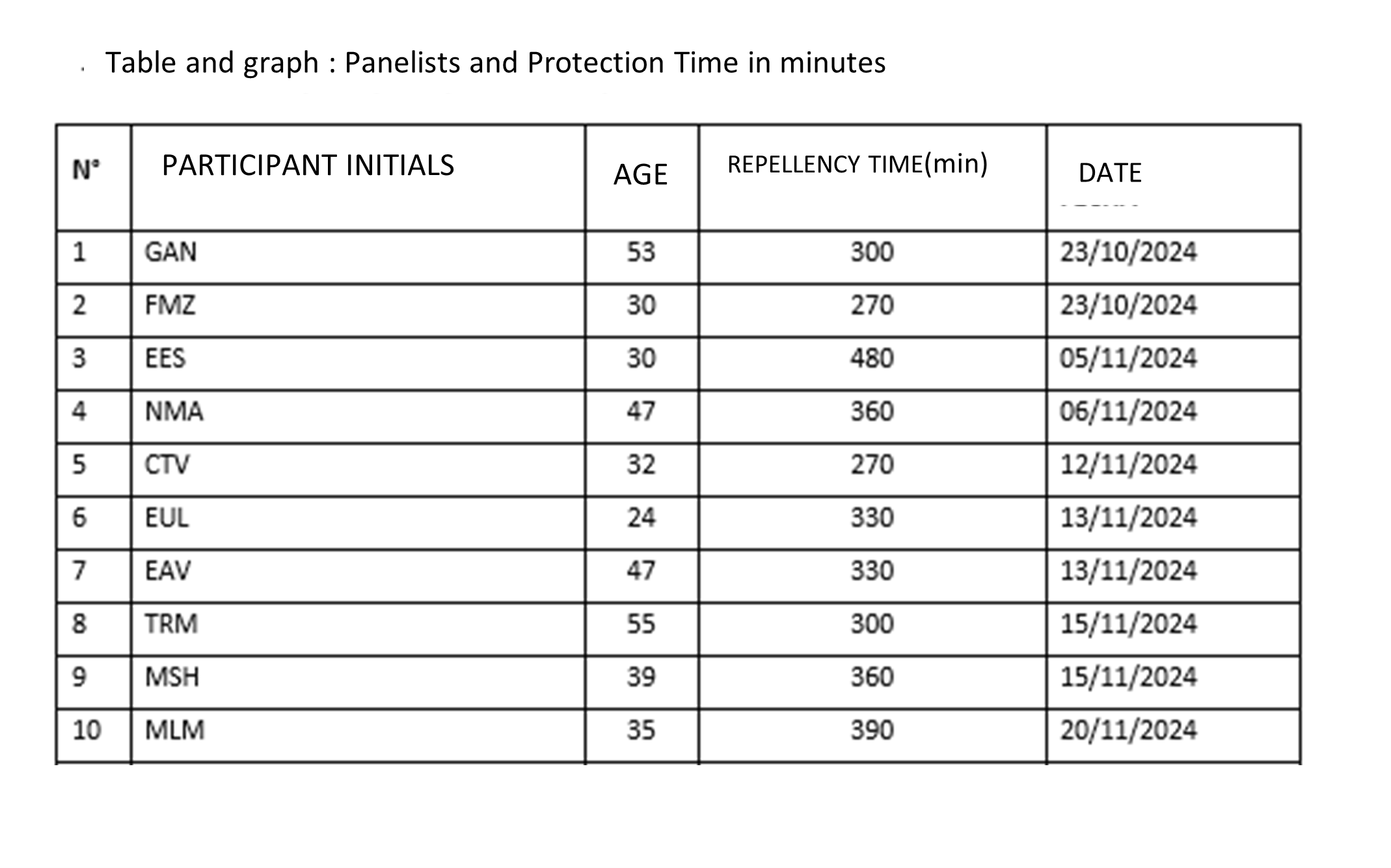

RESULTS TO SHARE
In conclusion, these in vivo tests will provide valuable information to manufacturers and raw material suppliers, helping to ensure that consumers receive reliable products.
Advertorial
CONTACT
Silvia H. Pérez Damonte PhD. Scientific Director CLAIM®







 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.