In Vitro Techniques in Preclinical Testing 1980-2013 by Ruth Roberts
6 October 2015
Codirector and Cofounder, specialising in integrated toxicology and ion channel expertise
We examined the use of in vitro (including in silico) techniques in preclinical safety testing by the pharmaceutical industry between 1980 and 2013 to determine patterns, drivers and challenges in uptake (Toxicol. Res., 2015,4, 1297-1307).
Data were collected via a survey sent to the Association of the British Pharmaceutical Industry (ABPI) member companies from the Nonclinical and Biological Discovery Expert Network (NaBDEN) requesting the number of compounds screened using in vitro and in silico tests at 5-year intervals between 1980 and 2005 then yearly from 2008 onwards. A utility score from 1 (poor) to 5 (excellent) for each assay was also requested.
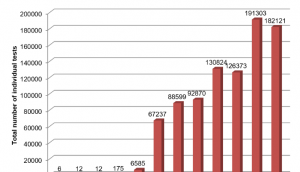
Four pharmaceutical companies and 3 contract research organisations (CROs) responded to the survey, providing >895000 data points across all years and all assays. Overall, there was a steady increase in the use of in vitro tests by the pharmaceutical industry between 1980 and 2013; indeed >20% of all in vitro tests reported were conducted in the last year of the survey window (2013) and >70% of all in vitro tests reported were conducted since 2010.
Use of in vitro tests peaked at >190000 tests per annum in 2012; >99% of this usage was in the three main areas reported of ADME, safety pharmacology and genotoxicity. Trends and step changes in uptake were most notable in the three main areas of ADME, safety pharmacology and genotoxicity and may be explained by the timing of adoption of the relevant International Committee on Harmonisation (ICH) guidelines.
Trends in uptake may also be explained by perceptions of utility where scores varied from poor (Eye Irritation – flourescein leakage) to excellent (Genotoxicity – Ames and Skin irritation – EpiSkin/Epiderm). In summary, the data show a large increase and a continuing upwards trend in development and adoption of in vitro alternatives to animal testing in pharmaceutical drug development providing new opportunities to improve success rates coupled with a strong commitment to the 3Rs.







 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.