Intertek evaluates the products impact on the cutaneous microbiota
12 April 2018
The cutaneous microbiota plays an essential role in skin health.
Consequently, it becomes necessary to address the evaluation of cosmetic impact and medical device on skin microbiota:
- Either to avoid any skin microbiota imbalance,
- Or, on the contrary, to reestablish the balance if any dysbiosis (atopy, dandruff, acne …).
Intertek conducts clinical studies on healthy volunteers or on volunteers suffering from a cutaneous pathology in order to analyze this impact.
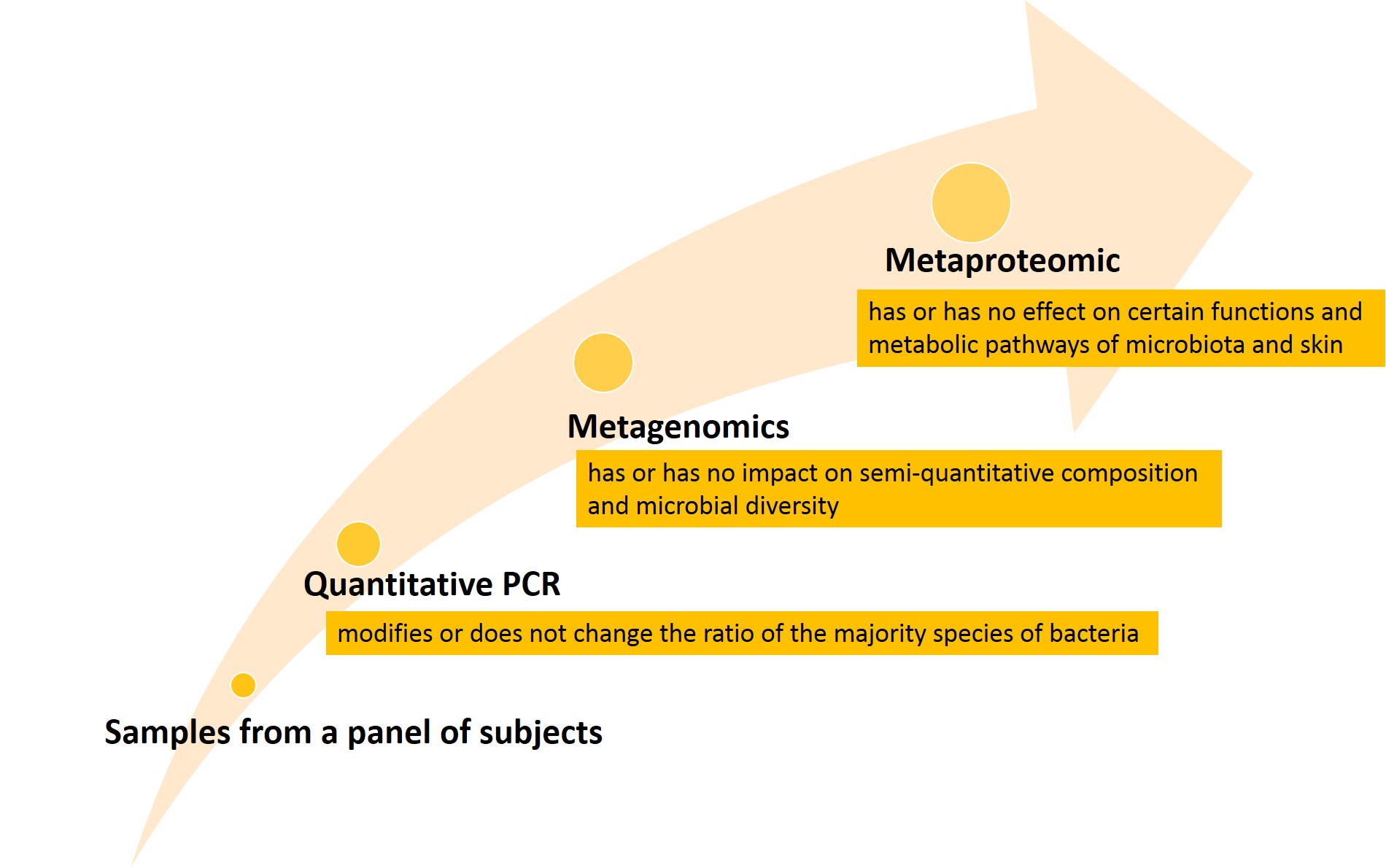
Intertek offers 3 levels of investigation:
Intertek’s expertise for Microbiota:
- A suitable recruitment of volunteers,
- Samples management expertise: sampling and transport,
- Analysis and results interpretation by skin microbiome experts team.
Intertek France – Clinical Research Department
48 Rue de la Colonie
75013 Paris – France
Email: france@intertek.com
Phone: +33 (0)2.78.94.03.78







 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.