Over the last ~60 years since William Russell and Rex Burch proposed the “3R’s”: Replacement, Reduction and Refinement of animal testing methods, many New Approach Methodologies have been developed to embrace these principles for most endpoints of interest to toxicologists and risk assessors, especially for personal care products and related raw materials. Although the results of many of these tests are used for internal purposes, such as research, development and due diligence evaluation of products, assays that have not received formal acceptance by intergovernmental organizations may not be suitable for regulatory submissions. Fortunately, there are many groups, public and private, that support these validation efforts to enable their use for compliance with regulatory mandates.
Last month, several new non-animal based OECD Test Guidelines were published by OECD, including: Defined Approaches for Skin Sensitization (OECD 497) and the In Vitro Reconstructed Human Epidermis Phototoxicity Test (OECD TG 498). Additionally, the OECD 442C guideline has been revised to include the kinetic Direct Peptide Reactivity Assay.
The RhE Phototoxicity assay takes advantage of benefits of a reconstructed human epidermis model in evaluating phototoxic responses after exposure to test materials that are challenging or otherwise incompatible with the 3T3 NRU assay. In addition to the newly published OECD test guideline, this assay is also cited in the International Conference on Harmonization (ICH) S10 guidance document; the results of the assay are suitable for Investigative New Drug submission applications to regulatory agencies party to this consortium.
The kinetic Direct Peptide Reactivity Assay (kDPRA), under the revised OECD 442C Test Guideline, permits resolution of category 1A (Strong) sensitizers from 1B (Weak)/non-classified substances. As an in chemico test, the kDPRA can be performed efficiently and cost-effectively. This assay has the advantage of evaluating the test material over multiple time-points and concentrations of test material in order to provide a peptide conjugation (a Key Event in the Skin Sensitization AOP) rate constant.
This value has been shown to be one of the most robust determinants in the evaluation of sensitization hazard1.
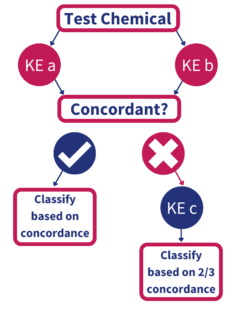
The Defined Approaches (DAs) to Skin Sensitization Test Guideline cites 2 strategies which can be used to evaluate hazard potential of compounds and to classify materials under the Global Harmonized System (GHS). One DA involves in vitro and in chemico testing and evaluation of multiple steps in the Adverse Outcome Pathway leading to skin sensitization. This “2 out of 3” approach will classify test materials as a GHS Category 1 sensitizer or non-sensitizer. (Fig, 1). The Kao-Brands developed “Integrated Testing Strategy” (Fig 2) has also been included in the OECD Test Guideline. This approach permits classification of sensitizers as strong (GHS 1A), weak (GHS 1B) or non-classified.
IIVS is proud to have been a part of the validation effort of these test guidelines as part of our mission to increase the use and acceptance of New Alternative Methods in Toxicology. Congratulations to all of our colleagues who made these test guidelines possible, including BASF SE, Givaudan, NICEATM, and Helena Kandarova, and to ICAPO which supports IIVS’ participation in the OECD expert work groups
Learn more about alternative approaches to non-animal testing by visiting our website: www.iivs.org or contact us directly: clientservices@iivs.org.
1 Natsch, A., Haupt, T., Wareing, B., Landsiedel, R., and Kolle, S.N., (2020) Predictivity of the kinetic direct peptide reactivity assay (kDPRA) for sensitizer potency assessment and subclassification. ALTEX, 37(4), 652-664
Figure 1: Schematic of the AOP-based “2 out of 3” defined approach. OECD TG methods for Key Events (KEs) 1, 2, or 3 are run in an undefined order until at least two of the three methods show consensus.
CONTACT
Brent Gilbert
Business Development Manager
30 W. Watkins Mill Road, Suite 100
Gaithersburg, MD 20878
Email: bgilbert@iivs.org | www.iivs.org
Personal Office: 813-598-5570