The COVID-19 pandemic – and the consequent confinement measures that were implemented by the governments – changed the clinical activity framework for the past 2 months, with some consequences that will be noticed for some more months, until a new vaccine or treatment appears.
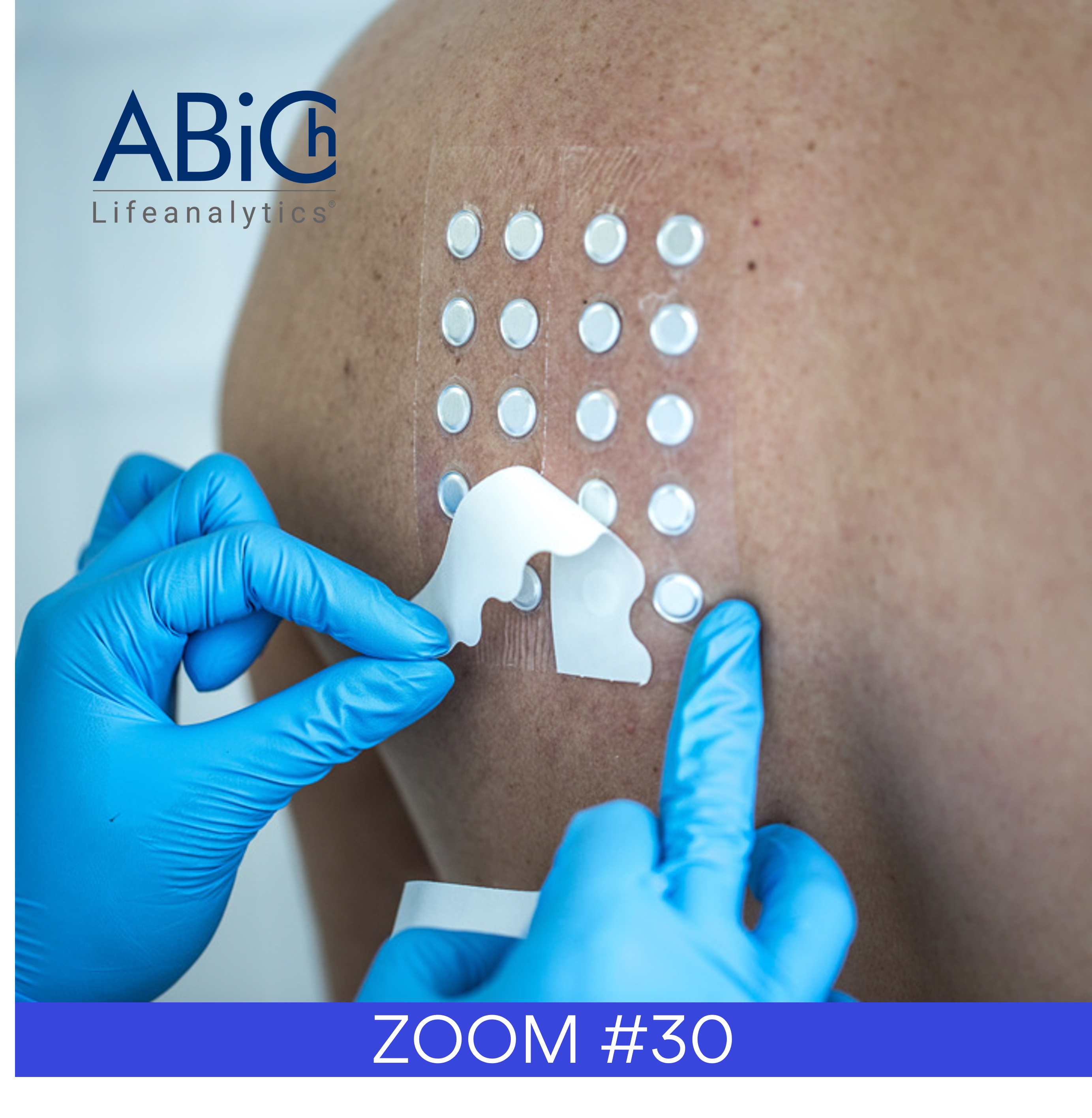
During this period PhD Trials®, a leading and innovative CRO in the cosmetic field, based in Portugal, was able to maintain its activity by implemented very restrictive hygiene and safety measures that ensured the safety of both our technical staff and all our subjects.
These measures assured that no one has being infected until this date and that the studies have continued, as some of you well know.
We express our gratitude to all our staff, subjects and clients that continue to rely in our work by maintaining the studies, continue to work in a difficult situation and continue to come to the facilities in a very particular environment.
A new normality has started and, therefore, we also wish to tell you that all our studies are open, including tolerance, in use studies, instrumental including biometrical studies and all the complex and advanced studies that we perform and that you well know.
Nevertheless, restrictions will be maintained, and the developed safety and hygiene measures will be part of our procedures in all the studies performed. Therefore we urge you to contact us in order to ensure your best schedule in this new normality that arrives, bearing in mind that we will continue the activity in order to guarantee that your studies are performed in all the safety conditions and with the quality you require.









 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.