Introduction
although skin care product candidates should be carefully tested for both safety and efficacy prior to being released on the market, far-reaching claims associated with the performance of these new products are frequently difficult to substantiate. This is primarily due to a lack of suitable technologies capable of accurately assessing alterations in skin properties.
- Safety implies that the products should neither damage skin nor create adverse conditions that include skin irritation and allergic responses (usually evidenced by the presence of erythema).
- Efficacy implies that the products should meet not only the manufacturers’ specifications but those of the users as well. Further, it is imperative that product efficacy be consistent with product claims.
Technology
polarization spectroscopy imaging is a technology that maps the microcirculation of the skin and other dermal properties by looking beneath the surface layer and capturing sub-epidermal images [1]. This technology, also known as Tissue Viability Imaging (TiVi), employs a methodology uniquely capable of profiling the selective light-absorption behavior of hemoglobin molecules present in red blood cells (RBCs).
These molecules absorb a significantly greater proportion of light in the green wavelength band compared to that of the surrounding tissue. In this respect, hemoglobin molecules are reliable biological markers for mapping and quantifying skin erythema and blanching.
The TiVi-technology is useful for assessing cosmetic and personal care safety, efficacy and consistency with regulatory guidelines.
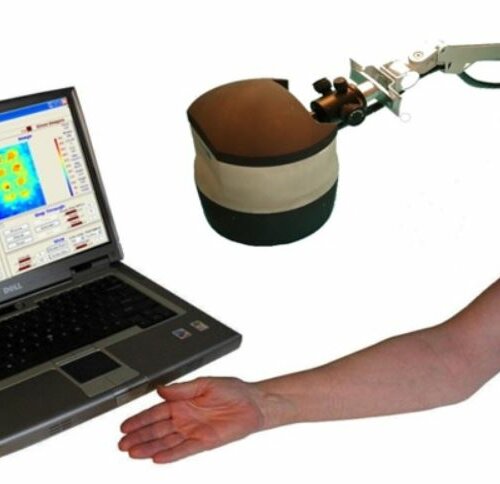
The TiVi700 2.0 camera is shown in Fig 1.
Photos and videos can be captured from the skin of the face, forearm or the back using the TiVi-camera – typically positioned 10 – 40 cm from the skin – controlled by the TiVi system software running on a PC or an AIO (All In One) computer. These photos and videos are converted to colour-coded microcirculation maps representing the local concentration of RBCs in the skin area under investigation.
Behind each pixel element in the map is a unique number that scales linearly with this local RBC concentration, which is used for quantitative analysis of skin erythema and blanching.
Applications
in a most frequent application, a skin care product candidate is to be tested for efficacy and safety over a predetermined period of time (for example 3 months). Prior to topical application of the substance on the skin (as a cream), baseline photos are captured. The test subjects then apply the cream daily throughout the test period. After termination of the test period the end-point photos are captured.
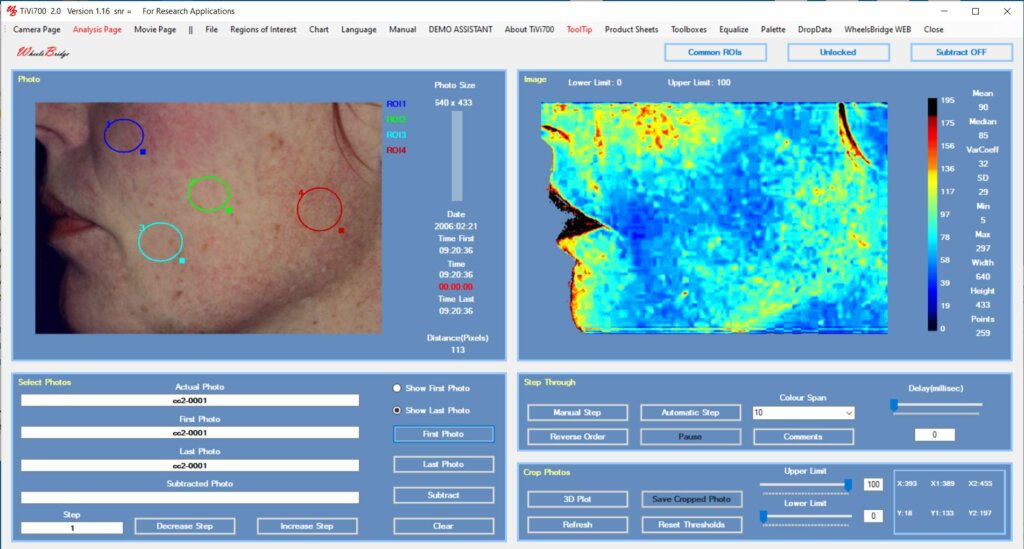
In the analysis, movable Regions of Interest (ROIs) are placed over the skin sections of interest and the average local RBC concentration is calculated. The data is automatically transferred to a table or spreadsheet for final analysis of alterations is RBC concentration depicting potential irritation of the skin caused by the substance under validation.
Preferably such as study should be performed using a double-blind approach with creams including and excluding the active substance in a random fashion. Fig 2 displays the TiVi-software user interface after application of ROIs.
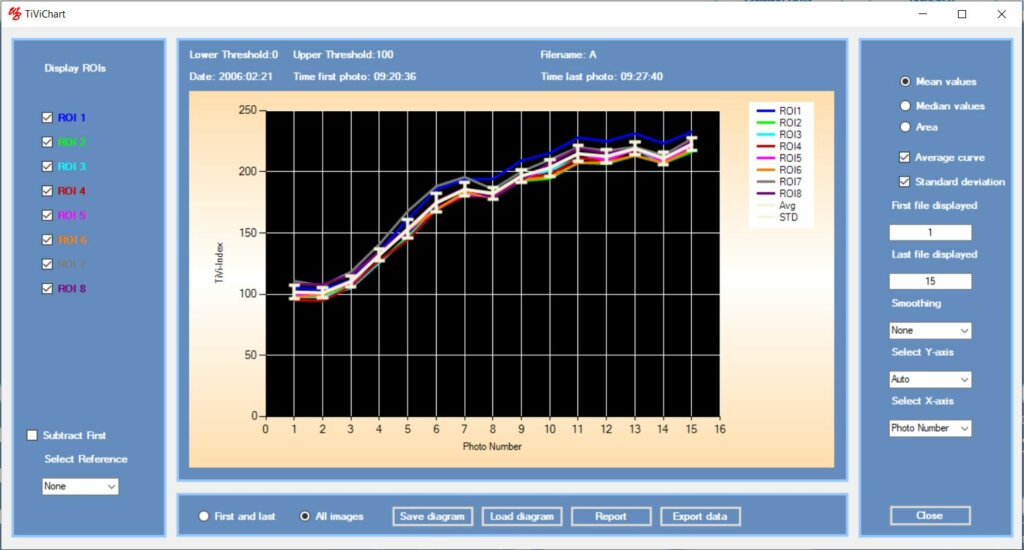
If the substance tested is expected to affect the microcirculation over a shorter time period (< 1 hour), the TiVi-system can be set to capture new photos at preselected intervals. After applying ROIs, time traces of the development of erythema or blanching are generated. Fig 3 shows the time traces for erythema development following topical application of methyl nicotinate using a Finn Chamber ® with eight patches over which the ROIs were drawn.
The average curve from individual test subjects can then be calculated and exported to a project window showing the individual average curves for all panelists in a single diagram. Such investigations frequently demonstrate that the peak value is reached after different elapsed time periods in different test subjects.
Visual scoring alone at a preselected point in time following application of the test substance may therefore not capture the individual peak responses accurately – the entire time traces need to be recorded to identify the individual peak responses.
In case the response time of the microvascular bed to an applied stimulus is shorter than a few minutes, the TiVi system should preferably be set to operate in video mode. In this mode of operating the maximum frame rate can be set to 30 frames per second.
TiVi Toolboxes – in addition to analysis of skin microcirculation, the TiVi-system uses optional toolboxes employing different software algorithms to investigate a variety of other skin parameters including analysis of 1) skin damage, 2) colour, 3) acne spots, 4) wrinkles, 5) pigmentation, 6) oxyhemoglobin trends, 7) small objects, 8) dimples, 9) sweat gland activity, 10) naevi, 11) wounds and 12) stubbles.
Conclusion
The ease of use of the Tissue Viability Imager TiVi700 2.0, makes it well suited for productive studies involving large panels off test subjects. The TiVi software enables user-independent analysis of data providing a strong foundation for product efficacy and claims substantiation. More information about the TiVi700 2.0 features can be found on the website www.wheelsbridge.com (Demo Videos and Tutorial page).
Reference – O´DOHERTY, J., HENRICSON, J., ANDERSON, C., LEAHY, M, NILSSON, G and SJOBERG, F. Sub-epidermal Imaging using Polarized Light Spectroscopy for Assessment of Skin Microcirculation. Skin Research and Technology 2007, 13; 472-484.
CONTACT
Phone: +46 708-765-190
E-mail: info@wheelsbridge.se










 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.