Mr Pellevoisin presents us the particular uses of SKinethic skin model to mimic epidermal invasion by human lymphocytes
The use of Skinethic reconstructed human Epidermis (RHE) to study immunocompetent cells trafficking and recruitment between skin compartments.
C.Pellevoisin1, G.Douillard2, F.Sahuc1, N.Ancellin2
1 – EPISKIN Academy, 4 av Alexander Fleming, 69366, Lyon cedex 7, France
2 – GSK Research Center, 27, avenue du Québec, 91951 les Ulis Cedex, France
Several skin diseases such as psoriasis or atopic dermatitis are associated with a chronic skin inflammatory state in which recruitment of specialized immunocompetent cells plays a central role. The ability to have an in vitro model able to reproduce the different steps of recruitment, activation, migration and interaction of these inflammatory cells with keratinocytes of a living epidermis could be very useful to better understand these pathologies and to develop new treatment able to target specific engagement steps.
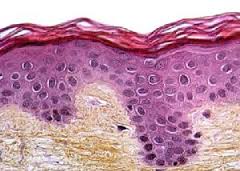
The SkinEthic RHE model is an in vitro epidermis reconstructed from human primary keratinocytes seeded on a polycarbonate membrane in a defined culture medium. This model, produced in an industrial scale, exhibits a high level of reproducibility and has been validated by ECVAM and OECD for the assessment of skin corrosion and skin irritation potential of chemicals. Such model is also widely used in dermatological research for its capacity to mimic human epidermal morphology and physiology.
To extend the fields of application of the SkinEthic RHE, we have developed a model with a modified insert to allow cell trafficking between the living epidermis and the underlying compartment. The membrane used is made of a microporous material for medium circulation with a low density of larger pores to allow cells migration. This new membrane patented by EPISKIN SA has been designed to limit the impact of such modification on the reconstruction of the epidermis as shown by the histological study.
Using this model, we’ve developed in collaboration with GSK a protocol to mimic epidermal invasion by human lymphocytes. The modified membrane of the SkinEthic RHE is incubated four hours with activated CD4 T cells (Dynabeads® Human T-Activator CD3/CD28). Twenty four hours later we observed by CD3/CD45 immunolabelling the infiltration of the CD4 T cells in the suprabasal layers of the epidermis.
The SkinEthicTM RHE migratory protocol opens the door to innovative methods to study complex interactions between living epidermis and inflammatory cells. Complementary studies are underway to confirm these preliminary results and will be completed by transcriptomics analysis to characterize keratinocytes answers.
Episkin.2016-may-SID-New-reconstructed-epidermis








 Follow us on Linkedin!
Follow us on Linkedin!