Key Factors that Influence the Most in Conducting Successful Clinical Research by Cliantha Research
14 June 2022
Clinical testing of cosmetics and personal care products play a key role in substantiating product claims for packaging and advertising whilst also ensuring that the marketed product is safe and effective for consumer use. Acquiring scientifically backed claims help commercial teams determine the positioning of the product. Clinically proven products are very well differentiated in the shelf from others. Product development is unambiguously the strong forte of research and development (R&D) companies, manufacturers and formulators. However, they require strong partners to clinically validate therapeutic claims of researched formulations. It is certainly the strong forte of experienced Clinical Research Organization (CRO).
Key Influencing Factors:
Managing a clinical trial involves a multitude of complex elements. Some key determining factors for conducting successful clinical trial include:
- Clinical Facility and Bio instruments
- Expertise of Organization and Investigator
- Previous Experience of Clinical Studies
- Regulatory Compliance
- Biostatistics, Data Collection and Data Management
- Development of Accurate Clinical Protocol
- Study Documentation & Report Writing
- Project Management
- Strong QMS System
- Adequate Trained Staff

72+ Years Rich Legacy of Cliantha Research
Cliantha Research, a full-service Clinical Research Organization (CRO), is a leading provider of Clinical research services, based in Ahmedabad, India. Cliantha’s mission is Science with Integrity. Cliantha has fifteen years of impeccable regulatory history with USFDA, WHO, MHRA, Health Canada, AGES, AEMPS, MCC, MOH, ANSM, MOPH, ANVISA, CAP, and NABL. Following 72+ years of legacy, 7285+ studies have been executed successfully under one flagship brand ‘Cliantha’.
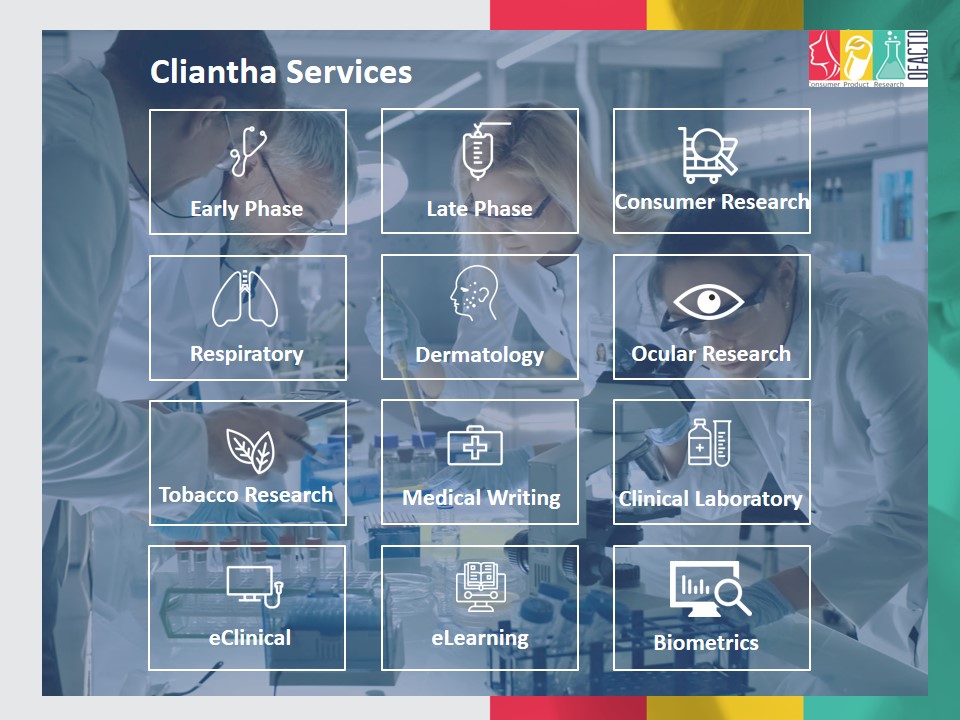
Cliantha Research is headquartered in India and has global presence in USA, Canada and Portugal. In last fifteen years, Cliantha has accumulated expertise in Early Phase (BA/BE), First in Man, Late Phase (various therapeutic areas), Respiratory, Tobacco Research, Dermatology, Consumer Research, Analytical lab, Diagnostic Central lab, IVRT, IVPT, Biometrics, Environmental Exposure Chambers, Pharmacovigilance and Medical Services.
Ofacto Consumer Research is a division of Cliantha Research Limited converging on consumer product testing. Our studies focus on product safety and efficacy, claim substantiation, consumer insights as well as marketing claim requirements. From the most standard to the most innovative, all our clinical trials are conducted according to the ICH GCP guidelines.
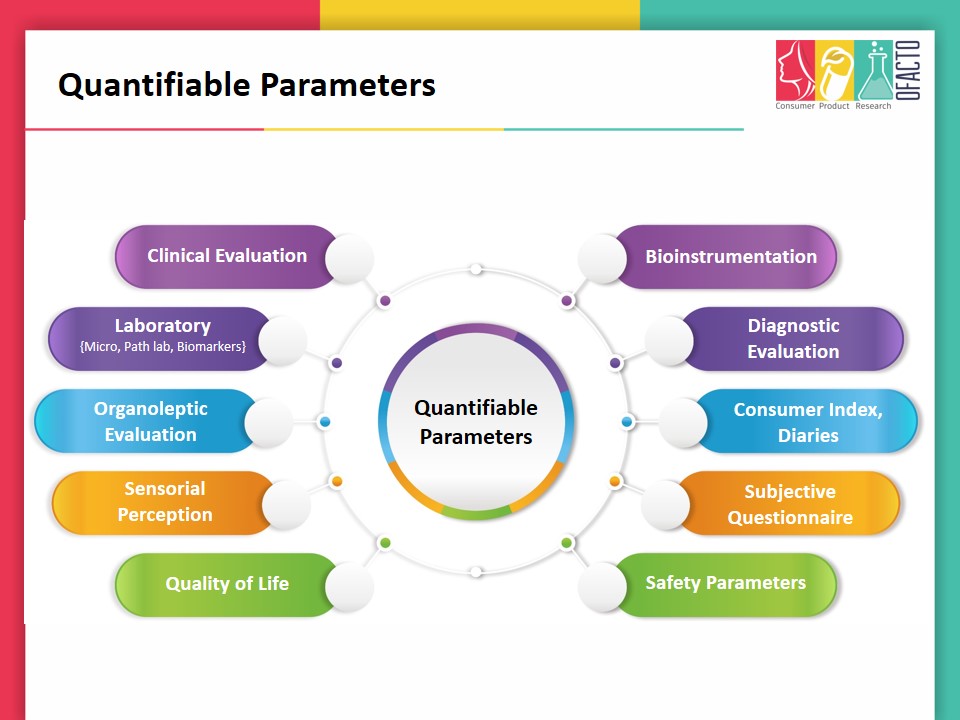
Ofacto has expertise in clinical testing since 1947, assuring complete data integrity and a quick turnaround time. Our clients include some of the biggest and most innovative global brands in personal care, cosmetic and nutraceutical industry. We offer total quality assurance expertise and support your new product innovation and development, by making sure that your claims are substantiated, and we become your preferred research partner in the consumer product space. Depending on the desired claims, Ofacto designs studies using quantifiable parameters i.e. Clinical evaluation, Bio-instrumentation, Quality Life Questionnaires, Laboratory {Micro, Path lab, Biomarkers, Histopath, Special Investigation i.e. Skin Biopsy/Ultrasound, etc.}, Consumer Index and Diaries.
Under Safety Testing, Primary Irritation Patch Test, Contact Sensitization (HRIPT), Cumulative Irritation Patch Test (CIPT), Occular Irritation Test, Phototoxicity Test, Comedogenecity Test, Mildness Testing and Safety in-use Test is being performed on regular basis. Duly equipped with advance clinical, microbiology and pathlab instruments, investigations are successfully executed in support of health claims viz. immunity booster, laxative, gut health, weight management, pain management, cardiac tonic, lipid lowering, glycemic index, diabetes, liver support, kidney support, joint support, energy booster, vitality & vigour, sexual wellness and cosmeceuticals etc. Ofacto also deals with safety evaluation and efficacy testing of functional foods, nutraceuticals, health supplements, OTC products, herbal medicines, dietary supplements, nutritional drinks, sugar-free products, botanical based, natural and herbal healthcare products. Label claims validation is performed for prebiotics, probiotics, multivitamin, multi-mineral, protein and gym supplements. Regulatory writing, specialized manuscripts writing and publications support is our other distinctive domains.
Depending on the desired claims, we provide different methods/techniques with a variety of measuring advanced scientific bio-instruments. We support client to choose the most appropriate technique using bio-instrumental or imaging methods to demonstrate the desired claims. The instruments used are well-known in the industry for decades and produced by well-known industries viz.
- Courage & Khazaka
- Canfield
- DiaStron
- Konica Minolta
- DermaLab
CASlite Nova – Phototrichogram, Fibra.One, VISIA CR, Corneometer CM 825, Cutometer Dual MPA 580, MoistureMeterSC, Tewameter TM 300, Vapometer, Glossymeter GL-200, Mexameter MX 18, Sebumeter SM-815, Skin pH meter, Spectrophotometer CM-2600d, Chromameter CR-400/410, AGE mu Reader, DandruffMeter, Halimeter, Pro Image Analysis Software, Environmental Controlled Hot/Cold Room, LUX meter, Body Composition Monitor etc. are some of our bioinstrumentation capabilities worth to mention.
Testing your product in a clinical trial generates scientific evidence for a product’s efficacy and safety. Clinical research organizations have an enormous, constructive influence on the process of new product development. They are inevitable fragment and hold the most critical factors of the process by successfully conducting clinical investigation. Explore their capabilities, take rounds of discussions with potential ones and choose clinical partners wisely.








 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.