Exclusive interview with Claire Blazy on the specific approach of the CIDP in preclinical and clinical evaluation services dedicated to the cosmetics industry
14 December 2022
- “For which claims does CIDP provide the cosmetic industry with an integrated approach to testing from in-vitro, ex-vivo to in-vivo?
CIDP is recognized in the cosmetics industry for the quality of its services and expertise. Our aim is to provide a full-service to our clients by proposing a wide range of tests:
- in vitro on primary or transformed monolayer-layer cell cultures,
- ex vivo on human skin explants and
- in vivo on human volunteers.
With the pandemic, several projects were hampered and following the resumption of our activities, we had to offer our clients agile and accelerated development programs. With our in vitro laboratory, ex vivo models and in vivo studies, we set up comprehensive development programs. We encourage our clients to add value to their data by publishing their data in scientific journals.
We therefore recommend our clients to opt for combined protocols to test their products on explants so as to gather preliminary data. In addition to being less expensive, this methodology, allows manufacturers to determine the inhibited or activated biochemical mechanisms and to adapt their strategy before moving on to clinical in vivo tests.
This approach can be adopted for a wide range of claims including cosmetic claims against exposome. A combination of in vitro/ex vivo/in vivo tests can be proposed to investigate the mechanisms triggered for healthy skin or pathological skin after exposure to the exposome. CIDP has developed ex vivo models with skin pathologies such as atopic dermatitis and psoriasis from human skin explants of healthy volunteers. The biological reaction of these explants can be observed under different conditions, including after exposure to environmental stressors such as ambient pollution, UV, blue light.



CIDP’s Preclinical lab and in vitro & ex vivo activities
- How does CIDP respond to brand inclusiveness trends through the multi-ethnic studies being increasingly sought after by brands?
Established in Mauritius since 2004, CIDP Mauritius has the advantage of having sunshine all year round and access to multi-ethnic population of all types of skin: from Caucasian to African, Asian, or Indian skin. Due to these attributes, the center in Mauritius specializes in photobiology studies and the pigment disorders that this entails. Through its network of aesthetic clinics, it also have access to skin explants from donors of different ethnicities, which allows as from the preclinical phase, evaluations on all skin types and phototypes.
In 2010, we opened a first subsidiary in Romania to have access to a panel of Caucasian skins who do not expose to the sun. Our center in Bucharest specializes in the evaluation of the the efficacy of suncare products. In 2011, we opened a third R&D center in New Delhi, India to give our clients access to a population of 25 million inhabitants in the city, which is ideal for studies requiring large cohorts of volunteers. Finally in 2012, we opened a subsidiary in Rio de Janeiro Brazil, the second largest consumer market of cosmetic products in the world. Brazil also has a multi-ethnic population that allows us to include cohorts of Caucasian, Hispanic, Asian, and African volunteers.
Our strategic location on all continents enables us to offer to the cosmetics industry the possibility to conduct multi-centric and multi-ethnic studies.



CIDP teams in Mauritius and Romania
- Efficacy testing for hair care products has been growing in recent years, what are the specific testing services offered by CIDP?
CIDP has developed different ex vivo and in vivo methodologies to substantiate claims for haircare products
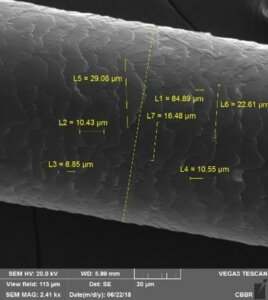
In ex vivo studies, CIDP proposes various assays on natural hair strands of different ethnic origins (Asian, African, Indian and Caucasian) to substantiate claims such as fiber resistance, brushing efficacy, shine, color lasting, hair growth or fall. Furthermore, the hair strands can be exposed to external stressors such as UV, air and water pollution and different biomarkers such as Malondialdehyde (MDA), Squalene, free fatty acids, the melanin and protein content, and Tryptophan degradation can be assessed. Scanning electron microscopy images can also be generated to evaluate the scaling of the hair fibers.


Hair strand exposed to UV and cigarette smoke
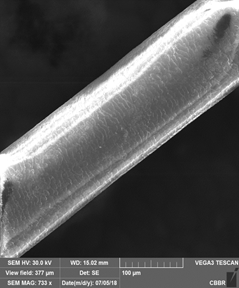
Unpolluted Hair Polluted Hair
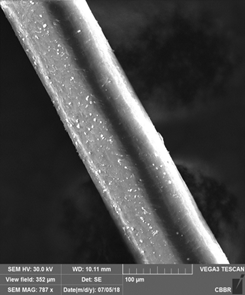

Unpolluted Hair Polluted Hair
In in vivo studies, CIDP proposes:
- various medical scales: dandruff, hair fall, hair breakage, hair shine,
- experts grading: elasticity, resistance, color efficacy, damage, repair, shine, straightening efficacy,
- biomarkers’ analysis: Squalene and Squalene monohydroperoxide, catalase, ceramides, inflammatory markers and Natural Moisturizing Factor (NMF),
- consumer’s evaluation including quality of life questionnaires,
- High-definition imaging with standard repositioning components is another tool that hair care specialist routinely incorporates in the study designs for substantiating clinical claims and providing images that can be used for marketing purposes.
Images obtained from SkinCam

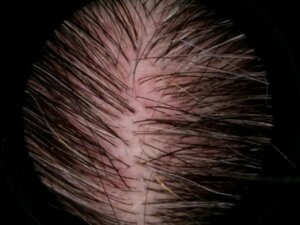
D0 D57
CIDP has also set-up new protocols involving real life sun exposure, real-life sea exposure, swimming-pool exposure, or even real-life sports activities to better support pioneering cosmetic claims. It offers the possibility for clients to combine both the ex vivo and in vivo techniques in a single study to derive the best results for their active ingredients or finished products.
- CIDP is one of the expert laboratories for anti-pollution studies, today, what is your approach to this subject?
Despite various cosmetics products being launched on anti-pollution claims, research work conducted on anti-pollution continues to be a real buzz within the cosmetic industry today. The rise in the need for the industry to answer to this issue remains the consumers increased awareness of the negative impact that pollutants have on the skin. Scientists in the field of cosmetology and dermatology, – concur that pollution is the second precursor of extrinsic skin ageing, right after UV. The real challenge of substantiating the anti-pollution claim is to be able to objectively show that a cosmetic product protects the skin against the harmful effect of air pollution. Moreover, as for any cosmetic claim substantiation, it is very important that consumers perceive the visual effect.
For the purpose, CIDP invested in a unique Controlled Pollution Exposure System, CIDP proposes quantified vaporization of pollutants (ambient dust, indoor dust, diesel, ozone) on the skin at controlled flux so as to show the effect of cosmetic products against the impact of these pollutants. Protocols have been set-up to demonstrate the barrier effect and the cleansing effect of cosmetic products against pollutants. The protocols have been designed to include a combination of biochemical evaluation, to demonstrate the effect of cosmetic products on pollutants biomarkers, and biophysical evaluation, to show the protective effect of cosmetic products on skin structures.
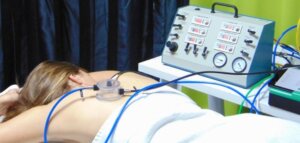
In vivo studies – The back of a volunteer exposed to a combination of pollutants using the Controlled Pollution Exposure System








 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.