Anticellulite Products: An Efficacy and Safety Testing Strategy by Princeton Consumer Research
3 August 2022
Cellulite is an aesthetic skin concern that affects 90% of post pubertal women and is not just specific to over-weight women. It manifests as a buildup of fat deposits beneath the skin that causes a lumpy, “cottage cheese” look on the thighs, rear end, hips, and belly. Cellulite is also known as orange-peel skin, due to its texture. It is characterized by large adipocytes that cause dimple contour alterations of the skin. There is reduced microcirculation, edema, localized adipocyte hypertrophy, extracellular matrix changes and abnormal elasticity and resilience of the skin.
Besides laser therapy, acoustic wave therapy, fillers and injectables, topical treatments are often used, with ingredients like caffeine, carnitine, retinol, escin from horse chestnut, ubiquinone and various herbal plant extracts and blends. It is important to understand that cellulite is complex occurrence and involves extended use of topical treatments, mechanical stimulation via massages besides adopting an active lifestyle.
Given the commercial interest in anticellulite topical products, efficacy, safety testing and patient satisfaction of these products is in demand for reducing the cottage cheese-like appearance of the cellulite prone areas and for silhouette contouring.
Besides noting circumference and body mass index, the following assessments are carried out:
- expert clinical grading
- measurement of skin hydration
- roughness
- elasticity with non-invasive bio instruments,
- subjective questionnaires,
- clinical photography.
Profilometry and various imaging modalities like ultrasound, laser doppler, thermography, MRI can be included. A good clinical design involves understanding cellulite etiology, knowledge of product ingredients, their concentrations and absorption, action and timeline of their effectiveness, and the utilization of sound scientific design and methodology.
A double blind, randomized placebo-controlled study of at least 10-12 weeks in duration with more subjects, use of standardized cellulite rating severity scales, same expert clinical grader throughout the study, non-invasive bio instruments for moisture, roughness, elasticity, validated clinical digital photography and ultrasonography or other imaging methods, along with self-perception questionnaires should be employed for capturing the severity of the condition and the treatment effects.
This combination approach with objective data can help in obtaining support or rejection of anticellulite efficacy claims for topical products.
Potential claims may include reduces appearance of cellulite; ameliorates the appearance of cellulite, reduces dimpling, reduces orange peel appearance, skin looks smoother, skin looks firmer, slimmer look, silhouette countering.
In conclusion efficacy and safety testing of anticellulite products with proven scientific designs, methodologies and proper statistical analysis will help provide their validation as well as help establish consumer confidence.
Cellulite grading
A cellulite severity scale, developed by Hexsel et al (2009) is a validated photo numeric scale with excellent reliability and internal consistency based on 5 morphologic aspects:
1) number of depressions,
2) depth of depressions,
3) clinical morphology
4) extent of skin laxity, flaccidity, or sagging
5) Nurnberger-Muller classification guide. Each variable is graded using three grades:
– Grade 1, or mild: There is an “orange-peel” appearance, with betTheyen 1 and 4 superficial depressions, and a slightly “draped” or sagging appearance to the skin.
– Grade 2, or moderate: There are between 5 and 9 medium-depth depressions, a “cottage cheese” appearance, and the skin appears moderately draped.
– Grade 3, or severe: There is a “mattress” appearance, with 10 or more deep depressions, and the skin is severely draped.
Baseline
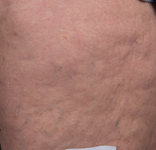
After Treatment
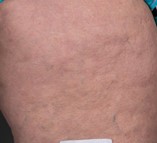
Reference: Hexel DM, Dal’Forno T, Hexcel CL. A validate photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2009; 23(5) 523-528
Discover Princeton Consumer Research profile and services on the Clinical Testing Platform
Read the entire FOCUS#6 on Slimming here
CONTACT
Dr. Nalini Kaul
Ph.D. Vice President Technical
Princeton Consumer Research








 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.