Safety and Tolerance Testing for the Cosmetics by Anne Charpentier
5 January 2023
After the first validations of the product toxicology, preservation (challenge test), stability comes the necessity to ensure the consumers safety. To evaluate the safety of cosmetics the first actor will be the toxicologist who will quantitatively and qualitatively review the formula composition of the product, then the testing laboratories will assure the safety assessment via in silico analysis, on chemicals, cells or on skin models. After the validation of the innocuity of the product, the studies of the tolerance can start on human.
In this article we will present the several ways, the most used, to evaluate the safety and the tolerance of cosmetics to make them safe for the consumers wherever there are used in the world.
Safety tests and the 3R principle
The cosmetics industry needs testing alternatives especially in safety to replace animal testing that are just not anymore ethically acceptable. For safety testing, in-silico, in-vitro or ex-vivo methods represent essential and reliable proof as predictors of the tolerance on human.
The safety of the cosmetic product is the first step in its evaluation. In Europe a report is required, in the form of the Product Information File (PIF), before it is placed on the market according to Regulation (EC) No 1223/2009 (Cosmetic Product Safety Report). This report on the safety of the cosmetic product includes a Part A on the safety of the product which gathers, among other things, information on the formula composition, its physico-chemical and microbiological characteristics, its stability, its toxicological profile. Part B is dedicated to the safety of the product and the conclusions of its evaluation carried out by toxicologist experts. Preclinical testing verifies the margins of safety for each ingredient and determines what tests are needed, analytical, preclinical, or clinical, to ensure the product safety.
Tests such as irritant, sensitization or photo toxicity potential can be evaluated by in-silico approach or (Q)SAR ((Quantitative) Structure-Activity Relationship). This analysis defines, via mathematical models, the correlation between a chemical structure and a biological or chemical activity.
Then, safety tests are conducted on chemical, cell cultures or 3D skin models, through standardized or innovative assays. European Centre for the Validation of Alternative Methods (ECVAM) has developed several OECD test guidelines to provide reliable and scientifically satisfactory standards for in vitro assays. For each specific target, valuable and numerous diagnostic methods are proposed offering a varied choice:
- Skin toxicity
- Corrosion: Electrical Resistance TER | RET [OECD 430], Corrosion Skin 3D Model [OECD TG 431], Corrositex [OECD 435]…
- Irritation: HET-CAM, MTT cytotoxicity, XTT cytotoxicity, Dermal Irritation ET50 …
- Sensitization: DPRA Direct Peptide Reactivity Assay [OECD 442C], Genomic categorization [Sens-is], H-CLAT [OECD 442E], U-SENS | IL-8 Luc [OECD 442], MTT- IL-8 [epiCS-SSPT], ARE-Nrf2 Luciferase KeratinoSens or Lusens Test [OECD 442D], Genomic categorization [GARDPotency OECD TGP 4.106], Genomic categorization [GARDSkin OECD TGP 1.406], ARE-Nrf2 Luciferase KeratinoSens or Lusens Test [OECD 442D], Combined approach [OECD 497] …
- Phototoxicity: 3T3 NRU [OECD 432], INVITTOX 121, OECD 498, Photo-hCLAT, Photo-Comet Assay …
- Photosensitization: Photo & Kinetic-DPRA Assay …
- Mucosa irritation: Irritation Assay System [OECD TG 496], Cellular viability [OECD TG 439], Zein solubilization test …
- Oral toxicity: OECD 129
- Eye irritation: Neutral Red, Fluorescein Leakage Test [OECD 460], Short Time Exposure [OECD 491], EpiOcular Eye Irritation Test [EIT] [OECD 492], Bovine Corneal Opacity and Permeability [adapted OECD 437], Cytotoxicity [OECD 492 Like], Agarose Overlay, Acute and repeated exposure, Isolated Chicken Eye [OECD 438], Ocular Irritation Assay System [OECD 496], Chorioallantoic Membrane Vascular Assay, NociOcular Assay, Vitrigel®-Eye Irritation Test [Vitrigel®-EIT] [OECD 494], Serious eye damage and eye Irritation [OECD 263], Acute Eye Irritation/Corrosion [OECD 405]
- Genotoxicity – Mutagenicity: Ames test [OECD 471], HPRT Gene mutation assay [OECD 476], Mammalian Cell Micronucleus Test [OECD 487], Genotoxicity, Comet Assay Mammalian Cell Gene Mutation [OECD 490], Micronucleus test, Chromosomal aberration test [OECD 473], Reconstructed Skin Micronucleus [RSMN], Adductomics, 3D skin Comet Assay.
For the skin sensitization test a combination of two in-vitro studies and an in-tubo test leads to hazard potential classification using an DA (defined approach). These studies target three different key events in an Adverse Outcome Pathway (AOP). Two concordant results lead to the classification as sensitizer/non-sensitizer (UN GHS 1 or NC). In addition to this the new OECD 497 provides an Integrated Test Strategy (ITS) based on these studies plus in silico prediction (e. g. QSAR or DEREK Nexus database) and allows GHS Classification into Potency subcategories 1A and 1B.
New Approach Methodology / Non-Animal Alternative Methods are now part of the routine toxicity testing of ingredients and cosmetics. These methods are a response to the 3R principle– the Replacement, Reduction and Refinement of animal experiments.
TOLERANCE, the essential evaluation on human
The tolerance assessments implemented on human subjects highlight the absence of irritant, sensitization, photo-irritant and sensitization potential on normal or reasonably foreseeable conditions of use.
The safety assessment is conducted by experts (doctor, toxicologist or equivalent qualified person authorized by the regulation). Depending on the country regulation and on the clinical study design (babies, ethnicities, repeated applications, sun exposure…), the protocols can be submitted to the ethics committee.
There are 4 categories of tests:
- Assessment of the irritation potential by Patch-Test
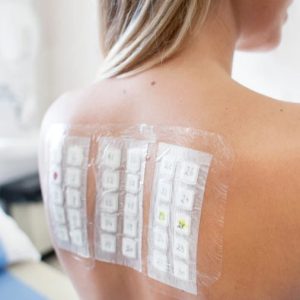
The patch test allows the study of skin tolerance by simple contact. It consists in a single application of the product, normally for 24, 48, 72 or 96 hours on volunteers under occlusive or semi-occlusive patch on the arm or the back. Then the outbreak of any skin reactions at patch removal (under medical or dermatological supervision).
- Use test under medical control: dermatologists, ophthalmologists, gynaecologist or ophthalmologist, paediatricians
- Use test with repeated applications under the normal conditions of use
- Repeat Open Application Test (ROAT)
- No comedogenicity
- Ocular projection or instillation
- Assessment of the allergenic potential
The sensitizing potential includes an induction phase, a resting phase, and a triggering phase
TCFS, Final clinical safety test, Human Repeat Insult Patch Test (HRIPT-Marzulli & Maibach)
- Photo tolerance: toxicity and sensitization
Clinical evaluation by scoring of the skin aspect after 1 single application and a UV exposure. One application during 24 h on 3 areas under occlusive or semi-occlusive patch. The conclusion regarding the product safety represents the final analyses of the data and the results of the cosmetics tests under several criteria: exposure, conditions of use, risks of misuse validated by a medical assessment.
The evaluation of the tolerance of the product dedicated to the sensitive skin is particular. The claim “sensitive skin” is possible if both of the following conditions are met:
- a) The volunteers included in the test of use carried out under normal conditions of use declared
recent and repeated history of functional symptomatology of skin discomfort (e.g., tingling, tightness, warm-up, itching, burning, redness…).
- b) These volunteers did not show an increase in symptomatology during the usual test
functional skin discomfort analyzed as relevant.
These tolerance testing on Human are one of the pilar of the safety of the product before its launch on the market. Depending on the type of products i.e. shampoo, hair dye, depilatory or skin care, the protocols have to be adapted in accordance with the usual way the product is applied i.e. ethnicity, rinse, frequency, quantity… It means that each evaluation manager must discuss the details of the protocol design with the toxicologist and each testing laboratory.
In conclusion, toxicity and tolerance ensure that consumers can use products without risk to their health. Human evaluation is the last step before in-depth studies of their efficacy and validation of their claims. Since the 2000s, toxicity tests have evolved very rapidly following advances in in-silico analysis technologies and the capabilities of artificial intelligence in data processing. In-vitro tests, on the other hand, are becoming more sophisticated with the use of increasingly complex skin models that can integrate ethnic, age and sensitivity variables. More and more sophisticated, also with the democratization of genomic and metabolomic analyses among others; the development of microfluidics techniques and the arrival of “Vegan” tests drastically reducing the use of animal by-products. Between guidelines and best practices, eco-responsible consumer expectations and scientific reality, the evolution of technologies will undoubtedly be a source for tomorrow’s tests.







 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.