Best Of #10 Virtual Clinical Trials, a new Way to Conduct your Studies by Datacapt
4 August 2021
For several years now, telehealth in medicine have been developing to facilitate patient access to care. Thanks to the health situation and based on telemedicine/telehealth, decentralized and virtual cosmetic clinical trials are developing.
They offer an organizational optimization in the management of:
- Timelines
- The number of subjects included
- Multi-center studies
These clinical trials in teleconsultation are envisaged when the CROs have the indispensable digital solution allowing:
- Digitalization of documents (eConsent, Case Report Form (eCRF), surveys (ePRO).
- The respect of security conditions in data management (Backups, Disaster Recovery Plan…)
- Compliance with the personal data collected
eConsult: A solution to conduct your clinical trials with Virtual Visits
This new approach, currently under development, responds to many requests from CROs because it:
- Accelerates the digitization of processes,
- Reduces the “costs” associated with travel, contributing to reduced pollution and lower energy consumption,A
- Allows for continuity of studies and follow-up of subjects even in pandemic situations.
- Offers the opportunity for multi-center studies and a larger panel
Datacapt eConsult provide:
- A secure nominative control of the subjects
- An integrated and secure high-definition video-consultation
- Explanation of the context and conduct of the study.
- The electronic signature of the consent with the video-consultation
- A personalized follow-up of each subject in video-consultation
Thus, although the study is done at a distance, the volunteer is really taken care of, which improves his engagement and compliance with the rules of the protocol.
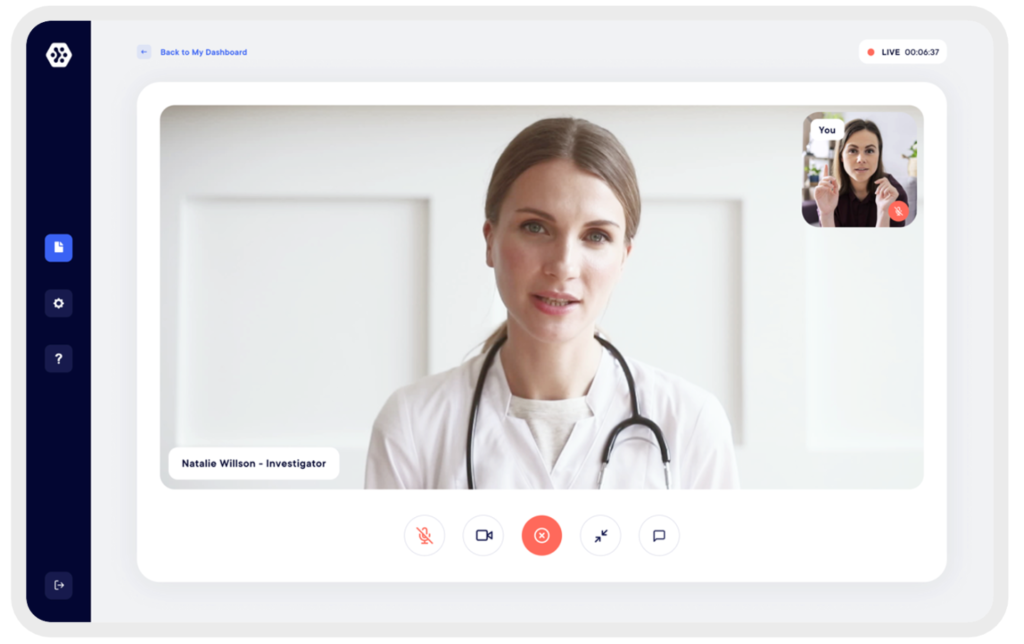
HD and secure tele-consultation directly integrated into the solution
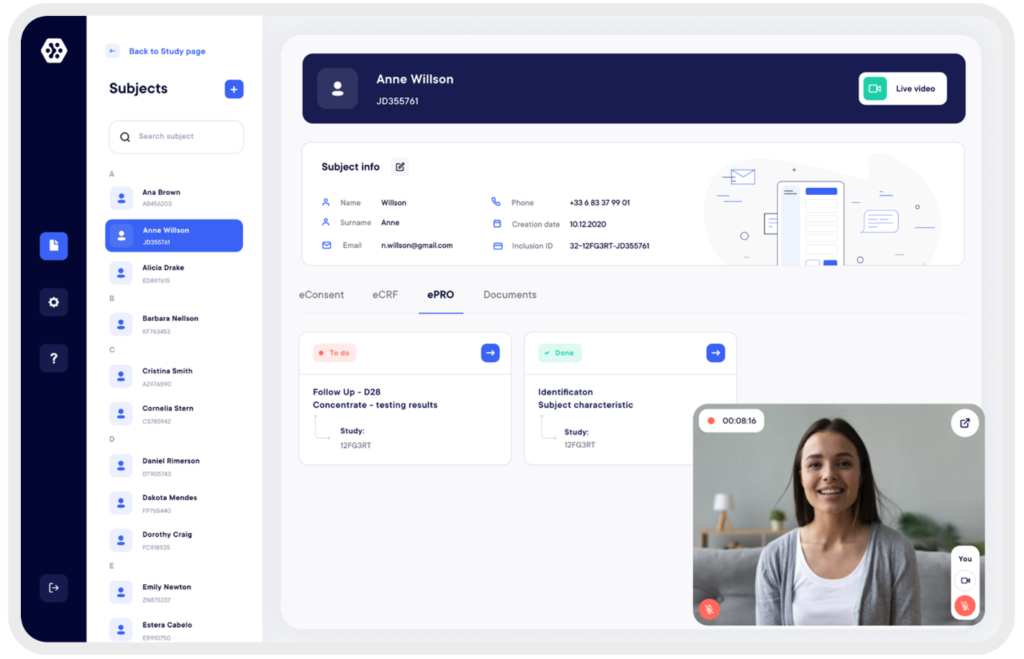
Investigators and technicians can have direct access to eConsent, eCRF and ePRO for each subject. Tracking of study tasks and progress is simplified. The documents exchanged between the subject and the investigator are secure and encrypted to ensure compliance and complete confidentiality of data.
Sponsor accesses can be opened to securely exchange study data.
Subjects Management: Investigators Access and Data Protection
A complete and adapted solution for your clinical trials
To respond to these constraints and make clinical tests in TeleVisit, decentralized or on-site in the best possible conditions, Datacapt offers modules that meet your needs:
- An eConsent that allows you to securely collect electronic consent from subjects while respecting the eIDAS standard and verifying the subject’s
- A new generation eCRF that is intuitive and without specific coding. Compliant with international security and quality standards, this module allows you to quickly build your eCRFs. Allows investigators to efficiently capture, store and process data on site or remotely in a standardized manner on one platform.
- An ePRO/eDiary, dedicated to the creation and management of your surveys and the capture of volunteers evaluations on mobile and tablet.
In terms of safety and compliance, our modules are validated: procedures, tests, validation report … and are compliant with the regulations and standards applicable to your clinical trials: RGDP, ICH GCP E6, 21 CFR PART 11, HDS (Health Data Hosting) …
Do not hesitate to contact us if you would like more information or a demonstration of the potential of our solution.
The Datacapt team
CONTACT
Florentin Ory
CEO
Tel. 06 77 38 02 61








 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.