Virus on the Skin by Syntivia and NeoVirTech
9 June 2020
NeoVirTech collaborates with Syntivia to develop a pipeline for the measurement of antiviral activities on living human skin explant
A strong partnership
NeoVirTech is a biotechnology company developing autofluorescent viruses for imaging and antiviral discovery. The company has developed the ANCHORTM technology that allows live cell imaging of virus infection, replication and propagation. The company has been highlighted by the PharmaTechOutlook magazine in the Top 10 drug discovery company in Europe in 2019.
Syntivia is specialized in the research & development of active ingredients or molecules in cosmetic and dermatology fields. We design and conducts preclinical evaluations to demonstrate the efficacy of active compounds.
A part of our expertise is based on our solid knowledge of skin physiology and our creativity in validating new study models.
The combination of our skills has enabled us to develop a model dedicated to the evaluation of products targeting viruses applied to the surface of the skin.
A proven study model
We have used living human skin samples and artificially contaminated them with an autofluorescent virus. Following skin adsorption and treatment with antiviral product we can quantify the number of infectious particles. In parallel, the impact of the product applied is evaluated on the skin structure and viability, providing an unprecedented relationship between viral disinfection potential and impact on the skin.

Protocol of virus and product application
This protocol gives a 24-hour answer on the antiviral effectiveness of your products on the skin.

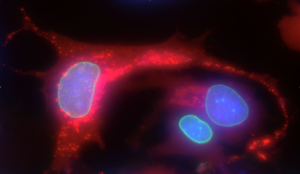
Quantification of infectious and replicating particles: Cells containing auto-fluorescent viral particles are quantified by automated microscopy (Thermo Scientific CellInsight CX7 high content screening microscope). More than 4000 cells are analyzed by sample.
The hand sanitizer we tested reduced the percentage of infected cells down to 3.5%. Our results show that it is crucial not to dilute hydroalcoholic solutions in order to preserve their efficacy on viruses on the surface of the skin.

Structural analysis of the skin explants after histological staining Masson trichrome (left) and immunohistochemistry Decorin in green, Elastin in red, Nuclei in blue (right).
Syntivia brings its expertise to analyse the effect of antiviral solutions on the human skin itself. Indeed, it is well known that the repeated daily use of sanitizers, de such as hydroalcoholic solutions, can deeply dehydrate the skin and create discomfort.
Our histological and immunochemical analysis show that the hydroalcoholic solution does not have a deleterious effect on the skin, which structural integrity is preserved.
We are glad to be able to offer tailored studies to our clients for the evaluation of their products, especially now that they are needed more than ever to ensure efficacy and user security. Do not hesitate to contact us for more information!
Contact information
Claire Leduc : Cleduc@syntivia.fr
Centre Pierre Potier, 1 Place Pierre Potier, Oncopôle entrée B
BP50624 – 31106 Toulouse Cedex 1
Tel : +33(0)974 770 660
Cell: +33(0)607 160 121








 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.