A GC-MS/MS analytical approach for regulatory compliance and safer products
Regulation (EC) No 1223/2009 governs the release of cosmetic products on the European market. It restricts the use of substances that may cause allergic reactions in a significant number of consumers.
The substances targeted are potential cosmetic allergens, identified by the Scientific Committee on Consumer Safety (SCCS).
To allow the regulation of these substances, it is necessary to include them in the DIP, product information file, and on the product label (FIL), visible to consumers.
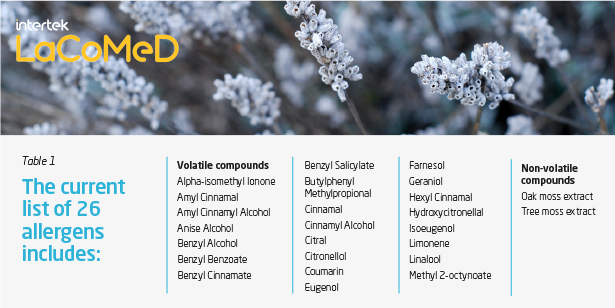
There are currently 26 potential allergens (Table 1) listed in Annex III of the Cosmetics Regulation that must be listed on the product label in the list of ingredients and comply with limits set according to the end use of the product:
- if their concentration exceeds 0.001% in unrinsed products
- if their concentration exceeds 0.01% in rinsed products
What is contact allergy?
Contact allergy involves a specific change in the reactivity of the immune system. Once contact allergy has developed, the cells of the immune system continue to recognize and react to the allergen. Therefore, symptoms such as allergic contact dermatitis may occur upon re-exposure to the allergen or fragrance allergens.
Extension of the scope of the determination of allergens
The Scientific Committee on Consumer Safety proposed in its 2012 opinion (SCCS/1459/11) that the consumer be informed of additional fragrance allergens present in cosmetic products and confirmed that the 26 allergens already listed in Annex II were still of concern.
Following this opinion of the SCCS, the European Commission published a public consultation in 20142 where it proposed to amend Annex III of the Cosmetics Regulation by including 61 additional contact allergens in addition to the 26 allergens already listed in Annex III. Currently, there is no published update to the list of potential allergens, however, the industry is preparing for changes that could be implemented in the coming weeks or months. If the expansion of the list is confirmed, cosmetic manufacturers will need to ensure that ingredients in products are properly identified and that labeling is revised to meet the latest expectations of the regulation.
Of these 87 substances, more than a third (30) are natural extracts, such as citrus sinensis bark oil and lavandin officinalis oil. These are still difficult to analyze, but can be identified by manufacturers and declared if present.
The remaining 57 chemically defined allergens (See Table 2 in the PDF version) are considered quantifiable by modern chromatographic techniques. The objective is to ensure that analytical methods are capable of providing reliable, sensitive and specific data, which are not affected by matrix effects and are suitable for an appropriate concentration range. There are several categories of samples, such as raw materials with low allergen content where the LOD/LOQ will be in the ppm range, or essential oils used in the manufacture of cosmetics with a quantification range from 20ppm to percent.
Sensitive, specific and fast determination methods
A rapid, sensitive and specific analytical method to identify and quantify the 57 volatile compounds is needed, but cosmetic samples present a particular technical challenge due to the complexity of the mixtures to be analyzed. Thus, the presence of interferents (molecules that may have the same affinity with the chromatography column used) may lead to the overestimation of an allergen (false positive).
Optimization by MRM
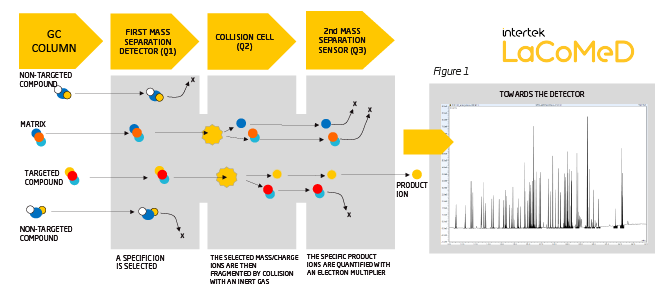
In order to avoid matrix effects and co-elution problems, our experts turned to GC-MS/MS (tandem mass spectrometry) with MRM (Multi Reaction Monitoring) technology.
In a simplified way: the analytes are separated from each other by the 60m GC capillary column and are introduced into the triple quadrupole mass detector (figure 1).
This allows the ion corresponding to the compound of interest to be targeted and, with the subsequent fragmentation of this target ion, a series of daughter ions are produced. One (or more) of these daughter ions can be selected for quantification.
Each of the 57 compounds has been the subject of particular attention, the transitions have been studied specifically for each allergen which has allowed a real improvement in the differentiation of the molecules, thus the MS/MS detection brings a real advance (see figure 2 and 3 in the PDF version) compared to the existing classical methods by GC/MS (simple quadrupole detection).
CASE STUDY
The study focused on the analysis of a sample of type «Essential Oil». It was analyzed using our GC-MS / MS method. We could observe that the complete chromatograph presented many peaks indicating a complex sample with potentially many allergens present.
Using our MRM approach, we performed a targeted assay on the allergens listed in Table 2. The retention time but also the transitions determined during the method development play an important role and they allow the analyst to confirm the presence or not of this allergen and thus to quantify it.
This measurement was performed in duplicate to ensure the accuracy of the result. Spiking can also be performed if matrix effects persist in order to obtain a reliable result.
How to Prepare for the Expansion of the Allergen List
It is recommended to start reviewing your raw materials and conducting allergen studies to maintain the competitive position and reputation of your product(s). As part of your raw material qualification programs, the LaCoMeD laboratory can support you in your approach with its analytical method for allergen detection, inspired by the IFRA standard with the added power of MS-MS detection.
Find out how to prepare to market your products in 6 steps by downloading the PDF version of our white paper: https://bit.ly/3fVTxDS
Conclusion
With the proposal to amend Annex III of the Cosmetics Regulation by including 61 additional contact allergens in addition to the 26 allergens already listed in Annex III, professionals in the cosmetics industry will have the responsibility to identify whether a product contains allergenic ingredients present in the new list. Developed to assist our customers in this transition, our new testing method is suitable for a wide range of concentration levels and is optimized for specificity, efficiency and accuracy. Our team is ready to help you secure your cosmetic products.
PDF version also available for download: https://bit.ly/3fVTxDS
CONTACT
+33 (0)3 85 99 12 80