Marisa MELONI – VITROSCREEN, MILAN, ITALY
Objective
In vitro Reconstructed Human Epidermis (RHE) possess a multilayered structure and display functionality close to the in vivo mechanism and predictive at both cellular and molecular levels. They represent a unique test system to study skin biology mechanism and to set-up experimental models to quantify ingredients and products efficacy. Biofilm formation is one of the most important virulence factors in infectious diseases. Biofilm-associated bacteria show an innate resistance to antibiotics, disinfectants, and clearance by host defences. These properties likely contribute to the persistence and recalcitrance to treatment of biofilm infections.
Among bacteria associated with wound biofilms, Staphylococcus aureus is one of the most common infectious bacterium and it has been shown that its presence causes delays in the healing process.
A specialized model based on 3D living in vitro RHE and methicillin resistant S. aureus (MRSA) and representing a stable biofilm phenotype has been developed. The model was useful to assess the efficacy of Chlorhexidine with a recognized antibacterial activity in eradicating the well-established biofilm.
In parallel, the skin compatibility of the antibiotic has been assessed on non-colonized RHE in the adopted experimental conditions compared to saline solution.
METHODS USED
Tissues have been superficially and gently scraped and then colonized with 2×106 cfu/tissue of S. aureus ATCC 33591.
1% Chlorhexidine has been directly and uniformly applied on the epidermis surface at 48h-52h-72h without product removal. No treatment was performed before 48h from infection to let the bacteria become adherent and produce a mature biofilm.
The efficacy of the product has been evaluated 4h after each treatment with the following parameters:
- Barrier function and fence properties by Trans Epithelial Electrical Resistance
- Microbiological counts on tissues homogenates and media
- Metabolic activity and cytotoxicity by ALAMAR BLUE assay
- Ultrastructural modification and S.aureus phenotype by Scanning Electron Microscopy (SEM) analysis
RESULTS OBTAINED
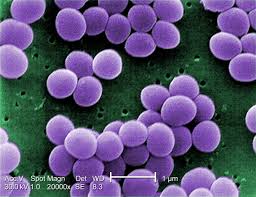
Chlorhexidine 1% treated tissues present at 52h and 56h a further decrease of the TEER values compared with colonized tissue, suggesting an additional reduction of barrier integrity after treatment linked to product activity where as at 76h it shows a severe barrier impairment (2%) that worsens after each treatment and high cytotoxicity, confirming its negative action on barrier and fence properties and cell viability. The positive control induced and maintained bacterial eradication, as demonstrated by bacteria count in the apical as well as in the homogenate and basolateral compartment. Repeated Chlorhexidine applications showed a damaged dehydrated epithelium surface with bacterial colonies on the surface, as it is possible to observe by SEM.
DISCUSSION
Treatments with Chlorhexidine caused a complete eradication of bacterial load of the planktonic state already after one treatment and of the adherent bacteria after two treatments. SEM analysis revealed the persistence of biofilm presence, but with a modified phenotype compared to the untreated control, with a dried-like structure, disrupted and composed of a non-viable bioburden.
CONCLUSION
In vitro model of biofilm presents several advantages compared to traditional in vitro assays.
M.MELONI SKINOBS JJPM 2015 .pptx
Contact
Marisa Meloni, PhD – CEO
Email: marisa.meloni@vitroscreen.com








 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.